Explanatory Memorandum
(Circulated by authority of the Minister for Health and Aged Care, the Hon Greg Hunt MP)REGULATION IMPACT STATEMENT
Background to mitochondrial disease and mitochondrial donation
Mitochondria are small DNA-containing structures in human cells. They produce 90 per cent of the energy that the body needs to function. Ordinarily, mitochondria are inherited almost exclusively through the maternal line (passed from a mother to her children), through the mitochondria present in the mother's egg cells.
A small proportion of an individual's genes (approximately 0.1 per cent) come from the mitochondria, and they are critical to the normal functioning of human cells. The remaining 99.9 per cent of an individual's genes come from the nuclear DNA, which informs appearance and personal characteristics.
Mitochondrial disease refers to a complex group of inherited conditions that can significantly lower an individual's health and life expectancy, and may be fatal. It is caused by changes to the mitochondrial DNA or nuclear DNA of an individual, which impact the ability of that individual's mitochondria to function properly. These diseases vary in presentation and severity, but common symptoms include developmental delays, seizures, weakness and fatigue, muscle pain, vision and hearing loss, multiple organ failure and heart problems; leading to morbidity and in severe cases, premature death.
The severity of symptoms and prognosis for the disease depends on a range of factors such as the overall impact on mitochondrial functioning, the number of mitochondria impacted and the way in which the affected mitochondria are distributed among an individual's tissues and organs.
The risk of developing serious illness due to mitochondrial disease is considered to be between one in 5,000 and one in 10,000. However, around one in 200 Australians are estimated to be predisposed to mitochondrial disease. In Australia, approximately 56 children are born each year with a severe form of the disease. The prognosis for these children is that most will die within their first five years.
Currently there is no known cure for mitochondrial diseases and treatment options are mostly limited to management of symptoms.
Mitochondrial donation
Mitochondrial donation is an assisted reproductive technology that, when combined with in-vitro fertilisation (IVF), has the potential to allow women whose mitochondria would predispose their potential children to mitochondrial disease, to have a biological child who does not inherit that predisposition. It involves a complex process to create an embryo which includes nuclear DNA from a man and the woman seeking to have a child (the prospective mother) and mitochondrial DNA from a different woman (the mitochondrial donor).
Mitochondrial donation can therefore minimise the risk of transmission of the prospective mother's mitochondria and in doing so aims to prevent future generations from inheriting these severe and debilitating diseases. Mitochondrial donation cannot however, be used to cure people with existing mitochondrial disease nor can it prevent mitochondrial disease caused by changes occurring in an individual's nuclear DNA.
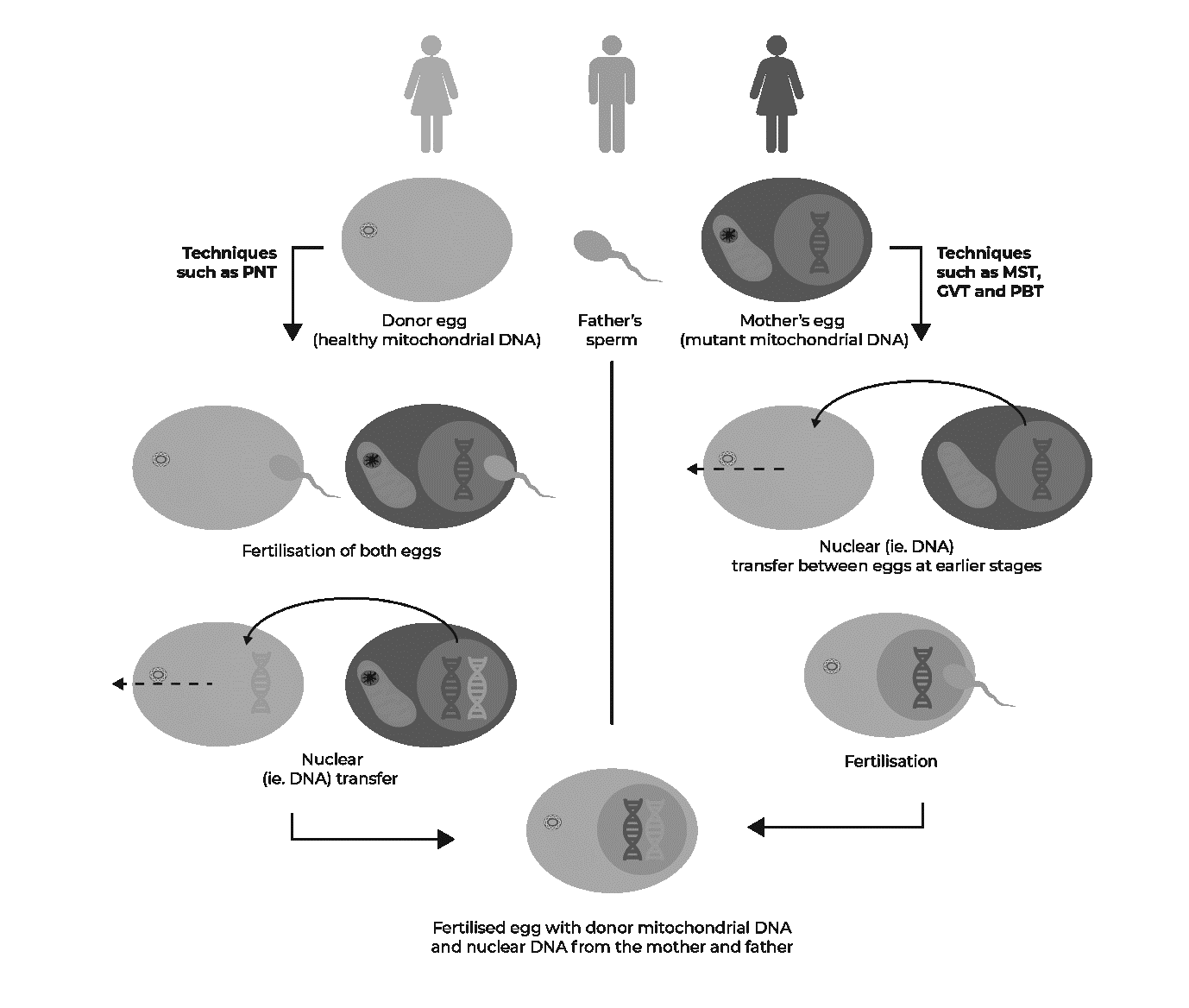
The science of mitochondrial donation is complex. The term 'mitochondrial donation' collectively refers to a number of specific techniques aimed at ensuring only healthy mitochondria are passed on to an embryo.
Only two mitochondrial donation techniques, Pronuclear Transfer (PNT) and Maternal Spindle Transfer (MST), are currently considered safe enough for use in clinical practice in the United Kingdom (UK), where mitochondrial donation has been legalised.
The following figure illustrates how the DNA from 3 individuals are combined using various mitochondrial donation processes.
Figure 1: The mitochondrial donation process
Interaction with the nationally consistent scheme
Currently mitochondrial donation techniques are prohibited in Australia under the Prohibition of Human Cloning for Reproduction Act 2002 (PHCR Act) and the Research Involving Human Embryos Act 2002 (RIHE Act).
In addition, in 2004 the Commonwealth and the states and territories agreed to the Research Involving Human Embryos and Prohibition of Human Cloning for Reproductive Purposes Intergovernmental Agreement (the IGA). [1]
The IGA creates a nationally-consistent scheme for the regulation of research involving human embryos and the prohibition of human cloning and some other practices. Under this scheme, all states and territories (other than the Northern Territory) have enacted corresponding laws that have the effect of achieving national consistency with the Commonwealth's PHCR and RIHE Acts. The IGA considers amendments to Commonwealth legislation that will not form part of the nationally consistent scheme (clauses 13 and 14).
Legalisation of mitochondrial donation under Commonwealth law would therefore require amendments to be made to both the PHCR Act and the RIHE Act. Furthermore, if mitochondrial donation was legalised for the limited purposes contemplated by the Bill, it would not be necessary for those amended provisions to form part of the nationally consistent scheme under the IGA. This would allow mitochondrial donation to be legalised in those limited circumstances through amendments made to the PHCR Act and the RIHE Act without corresponding amendments needing to be made to state or territory law.
Consistent with the existing regulatory scheme, when legalised, mitochondrial donation would be regulated by the National Health and Medical Research Council (NHMRC) Embryo Research Licensing Committee (ERLC). The ERLC has a range of prescribed functions to ensure tight control of research involving human embryos. [2] This approach would be in line with the regulatory framework that has been implemented in the United Kingdom for mitochondrial donation.
Previous examinations of mitochondrial donation
In 2018, the Senate Community Affairs References Committee undertook an inquiry into the Science of Mitochondrial Donation and Related Matters (the Senate Inquiry). [3] The Senate Inquiry looked at the impacts of mitochondrial disease, the science of mitochondrial donation, legal and ethical considerations and regulation. The final report arising from the Senate Inquiry recommended that some further consultation should be undertaken with the community, relevant experts and the states and territories before mitochondrial donation was introduced into Australian clinical practice. [4] In 2019-20, the NHMRC undertook a series of community and expert consultation activities in response to the Senate Inquiry recommendations. [5]
The consultations undertaken in Australia followed on from similar, extensive, consultations undertaken by the UK Human Fertilisation and Embryology Authority (HFEA) prior to the legalisation of mitochondrial donation in the UK. HFEA conducted four separate scientific reviews of the safety and efficacy of mitochondrial donation techniques (in 2011, 2013, 2014 and 2016), which led to an expert panel recommending PNT and MST as the two techniques for cautious adoption into clinical practice. [6] In addition, the HFEA undertook two public consultation processes: one on the ethical issues raised by mitochondrial donation in 2011-12; [7] and the other on the proposed draft regulations for mitochondrial donation in 2014 [8] .
RIS question 1: What is the policy problem you are trying to solve?
This proposal examines options to minimise the risk of children inheriting mitochondria from their mothers that would predispose those children to severe mitochondrial disease.
In Australia, approximately 56 children are born each year with a severe form of mitochondrial disease and most will die within their first five years. [9] Some of these instances could be prevented if mitochondrial donation was legalised in Australia for the purpose of minimising this risk.
As noted above, mitochondrial donation is currently prohibited for use in Australia under the PHCR Act and the RIHE Act, and corresponding state and territory laws. Unless the existing legislation is amended to legalise mitochondrial donation in Australia, affected women will not be able to access this technology in Australia and will be prevented from having their own biological children who are not predisposed to severe and debilitating mitochondrial disease.
The risks for children born using these techniques are not yet fully understood and the available scientific evidence to support this procedure is limited. This is due to the small number of births globally and restrictions around patient privacy which limits the sharing and publication of data. The implications for subsequent generations also remain unknown.
Public consultation on mitochondrial donation undertaken in Australia, has highlighted that some people in the community are concerned about the potential legalisation of mitochondrial donation in Australia. The NHMRC and Senate Inquiry processes identified a number of sensitivities, including a number of ethical issues and safety concerns.
For some in the community these ethical issues are highly personal and significant, preventing support of mitochondrial donation, despite its potential significant benefits.
The Australian Government is aware of these sensitivities and is seeking to introduce mitochondrial donation which finds a balance between helping couples to have biological children who are not at risk of mitochondrial disease while managing other risks and minimising potential harms.
Any proposed approach for Australia will also require prospective parents seeking access to this technology to attend pre-treatment counselling, where alternative options and the potential risks of mitochondrial donation will be fully explained. This approach will allow for parents to make their own informed decisions, and provides for reproductive choice.
International status of mitochondrial donation
In 2015, regulations were passed in the UK approving mitochondrial donation to be used for human reproductive purposes to prevent the transmission of serious mitochondrial DNA disease. [10]
Access to mitochondrial donation is tightly regulated in the UK. Facilities wishing to provide the service and prospective parents wishing to access it must both meet strict licensing conditions. Permitted techniques include MST and PNT, which are considered to be safe enough to be used for human reproductive purposes. For prospective parents, conditions for approval are based on clinical considerations such as the specific mitochondrial DNA changes carried by the mother (linked to likelihood of passing on severe mitochondrial disease), the percentage of impacted mitochondria and evidence that this is the only option for having a biological child without passing on severe disease.
Currently, only one facility has been licenced, and the licence only allows for the use of PNT [11] . As of November 2020, it is understood that up to 21 couples had attained a licence to receive treatment and up to 8 treatments had been approved. However, the outcomes of these treatments have not been made publicly available for reasons of privacy.
Other countries such as the United States of America (USA) and Canada do not currently allow the technique. [12] The Japanese government recently announced that it will lift its ban on the nuclear transfer of a fertilised egg for the purposes of research (but not for reproductive purposes) into mitochondrial disease. [13]
A small number of children are reported to have been born following the application of mitochondrial donation techniques in countries where these techniques are not currently prohibited including, for example, Mexico, Greece and the Ukraine. [14]
RIS question 2: Why is Government action needed?
There is currently no known cure for mitochondrial disease and treatment options are mostly limited to the management of symptoms. Mitochondrial donation offers the only means of minimising the risk of a woman whose mitochondria would predispose the woman's offspring to mitochondrial disease, having a biological child who did not inherit this predisposition.
As noted above, previous reviews into mitochondrial donation have identified the need for the Australian Government to examine options to mitigate the impact of mitochondrial disease on families, including through the consideration of pathways to legalise mitochondrial donation techniques.
Given that mitochondrial donation techniques are currently prohibited in Australia under Commonwealth laws, action by the Australian Government, rather than by state or territory Governments, would be needed to make this technology legally available. It should also be noted that any Australians seeking to travel to the United Kingdom to access this treatment are significantly inhibited from doing so at present, given the risks associated with the COVID-19 pandemic. It is also understood that this is not currently being offered as an option by the only licensed clinic in the UK and that this position is unlikely to change in the near future.
RIS Question 3: What policy options you are considering?
This RIS presents 3 options.
Option 1 - Maintain the status quo
Under this option there would be no future pathway to make mitochondrial donation available in Australia.
Affected women would not be able to access mitochondrial donation techniques in Australia but may still choose to have a biological child who may be impacted by mitochondrial disease. Alternatively, they may try to access the mitochondrial donation procedure overseas, or they may pursue alternative options to have a non-biological child such as adoption.
Option 2 - Legalise mitochondrial donation as a pathway to clinical use
Two alternatives to amend the existing legislation and regulations to legalise mitochondrial donation in Australia are presented below. While both result in the possibility of mitochondrial donation being made available in select clinical settings, they are differentiated by the speed and process they follow to reach this outcome.
Option 2A
This option would introduce mitochondrial donation through a staged process, which would provide for a cautious introduction of the technology. This approach would initially legalise mitochondrial donation for use in research settings and through a clinical trial for human reproductive purposes to determine the safety, efficacy and feasibility of mitochondrial donation for reducing the risk of transmission of serious mitochondrial disease in humans (Stage 1), before permitting its application in clinical practice more broadly under Stage 2.
Transition to Stage 2 would be a decision of the Commonwealth Government, following consideration of the progress and outcomes of the trial, and other expert advice.
Under this option, the PHCR Act and the RIHE Act would be amended to legalise mitochondrial donation under Commonwealth law for the purposes of pre-clinical research and training, and for a clinical trial of a specified mitochondrial donation technique, including associated research and training. Regulation and monitoring of mitochondrial donation licences would be undertaken by the ERLC in line with current processes for monitoring research involving human embryos but expanded to include specific mitochondrial donation licences.
To enable transition to Stage 2, the PHCR Act and the RIHE Act would also be amended to legalise mitochondrial donation for clinical practice and associated research and training using permitted mitochondrial donation techniques. However, organisations will not be able to apply to the ERLC for either of the two clinical practice related licences until a particular technique is specified in the Regulations for this purpose. Permitted techniques for clinical practice will not be specified until Stage 1 has been completed and the safety and efficacy of the mitochondrial donation technique has been demonstrated.
It is also not necessary that the amended provisions form part of the nationally consistent scheme under the IGA. This is because regulation of the use of mitochondrial donation techniques in clinical practice in a particular state or territory would be an aspect of the general regulation of assisted reproductive technologies in that state or territory. States and territories generally are responsible for regulating assisted reproductive technology in their jurisdiction, and the regulation is generally not consistent across Australia. As a result, clinical practice in the use of mitochondrial donation techniques will not need to be nationally consistent, and will not need to form a part of the nationally consistent scheme under the IGA.
However, it will be open to states and territories, together with the Commonwealth, to agree to regulate clinical practice in mitochondrial donation in a nationally consistent way in the future, should they choose to do so prior to the transition to Stage 2.
Further detail on the proposed stages is provided below:
Stage 1 (research and clinical trial)
- •
- The ERLC of the NHMRC will regulate mitochondrial donation in Australia. To facilitate this, the role and remit of the ERLC, will be expanded to include licensing and oversight of research and training licences, and clinical trial licences required to use mitochondrial donation techniques on human embryos.
- •
- The ERLC will initially develop the administrative requirements and assessment procedures for those individuals and organisations applying for one of 3 licence types allowing for mitochondrial donation in Australia:
- 1.
- Pre-clinical research and training: lab-based research only, not including research and training in preparation for a clinical trial
- 2.
- Clinical trial research and training: research and training in preparation for a clinical trial
- 3.
- Clinical trial: licence allowing for mitochondrial donation for reproductive purposes, in a clinical trial setting.
- •
- The ERLC and NHMRC will also develop processes for monitoring licence holders, which will include desktop and on-site inspections by NHMRC inspectors.
- •
- The Commonwealth Department of Health will run a competitive grant process to identify a suitable organisation to run a clinical trial under stage 1.
- •
- It is expected this trial will run for around 10 years. While there is a relatively small number of women that may be assisted through the trial, it will not be a fast process due to the potential for participants to require multiple IVF procedures before a successful pregnancy is achieved.
Transition from Stage 1 to Stage 2
As noted above, transition to clinical practice using a specified mitochondrial donation technique under Stage 2 would commence only after mitochondrial donation techniques suitable for use in clinical practice have been prescribed in the Research Involving Human Embryo Regulations 2017.
This decision will be made taking into consideration the outcomes of the clinical trial, and other expert advice.
Stage 2 (clinical practice)
At this stage, jurisdictions may choose to opt-in to the national regulatory framework, which will allow for licenced clinical practice, in participating states or territories.
As part of the regulatory scheme, two additional licences overseen by the ERLC, will be introduced in addition to the licences available in Stage 1, including:
- 1.
- Clinical practice research and training: allows an accredited clinic to prepare for using the permitted technique in a clinical practice setting
- 2.
- Clinical practice: would allow the use of specified mitochondrial donation techniques in a clinical practice setting.
Option 2B
This option would permit mitochondrial donation to be introduced into clinical practice for reproductive purposes, with the aim of reducing the risk of transmission of serious mitochondrial disease in humans.
In effect, this option would skip Stage 1 (above), which is focused on further research and a clinical trial, and go straight to a national model that allows for mitochondrial donation to be offered in clinical practice.
Once introduced into clinical practice, organisations (such as IVF clinics) would be able to apply for a licence to offer this procedure, which is currently prohibited, as part of their business model.
The use of specified mitochondrial donation techniques for research and training and clinical practice would be subject to the same strict licensing conditions overseen by the ERLC as outlined in Stage 2 above.
Option 3 - Support access to overseas treatment options
This option was raised in the Senate Inquiry, and would see the Australian Government providing assistance for impacted couples to access mitochondrial donation in countries such as the UK where the procedure has been legalised and is well regulated. Couples could also choose to seek their own treatment outside of Australia, however this would likely be a health and financial risk if it was not in a country where the procedure was carefully regulated.
Government support for couples to access mitochondrial donation overseas is not currently available. However, a Medical Treatment Overseas Program is administered by Government, which provides financial assistance for Australians with life-threatening medical conditions to receive proven life-saving medical treatment overseas where effective treatment is not available in Australia. Mitochondrial donation would not meet the existing mandatory medical criteria, and should this option be pursued, consideration would need to be given to the merit in expanding the criteria beyond life-threatening conditions.
RIS Question 4: What is the likely net benefit of each option?
Option 1 - Maintain the status quo
This option means that impacted couples would not be able to access mitochondrial donation techniques in Australia. They but may still choose to have a biological child who is impacted by mitochondrial disease, seek to access the procedure offshore or pursue alternative options to have a non-biological child such as adoption.
Under this option a number of children are likely to continue to be born in Australia each year with severe mitochondrial disease, and with a life expectancy of less than five years.
There is no regulatory impact, or cost, associated with maintaining the status quo. There is also no net benefit associated with this option.
Option 2 - Legalise mitochondrial donation as a pathway to clinical use
Legalising mitochondrial donation in Australia would require the establishment of an appropriate regulatory and licensing framework to ensure access to the procedure is carefully controlled, and conducted safely and ethically by licenced facilities with suitably qualified expertise. The proposed framework outlined below (and detailed at Attachment A) has been largely modelled on the approach undertaken in the UK and adapted to existing mechanisms that are aligned to the Australian system.
The proposed Australian regulatory framework and licensing requirements will have a regulatory impact on the organisations wishing to offer mitochondrial donation, the families wishing to access the technology and the ERLC, the body responsible for administering the licensing system.
Option 2A
For Option 2A, during Stage 1, organisations wishing to undertake a clinical trial using mitochondrial donation techniques for human reproductive purposes will be required to apply to the ERLC for two separate, consecutive licences: an initial clinical trial research and training licence followed by a clinical trial licence.
To receive Government funding for the trial, the organisation will also need to apply for and be successfully awarded a Commonwealth clinical trial grant. It is expected that only a small number of organisations (1 - 3) will be interested in undertaking the clinical trial. It is also intended that Commonwealth funding will be approved for one clinical trial under Stage 1. Therefore, only one organisation will be likely to apply for a clinical trial research and training licence and a clinical trial licence.
The third category of licence, a pre-clinical research and training licence, under Stage 1 is separate and will only permit research and training to be undertaken for non-reproductive purposes, without a view to conducting a particular clinical trial. It is expected that only a very small number of organisations (up to 5) may wish to apply for this type of licence.
Under Stage 2, any clinics located in states and territories that opt-in to the national regulatory framework governing the broader clinical provision of mitochondrial donation, who wish to offer mitochondrial donation, will be required to apply to the ERLC for a clinical practice research and training licence followed by a clinical practice licence.
Individuals wanting to access the procedure through the clinical trial or through Stage 2 will also need to apply and seek approval from the ERLC that they are appropriate candidates for mitochondrial donation.
Option 2A, also allows for a rigorous clinical trial of this technology in Australia under Stage 1, so that detailed knowledge about the technique is known to Australian policy developers and regulators prior to its broader introduction into clinical practice. This approach allows for the development of knowledge to ensure that the safest and most effective use of the technology can be ascertained before it is made more generally available under clinical practice. This will also ensure that the medium and some of the longer-term risks will be known and can be assessed and managed appropriately.
As detailed at Attachment B, the total regulatory costs associated with Option 2A
(Stage 1 and 2) is $188,857 annually or $1,888,568 over ten years. This cost will be incurred by businesses.
Option 2B
For Option 2B, which proceeds straight to clinical provision of mitochondrial donation, the regulatory costs associated with Stage 1 of Option 2A would not be incurred.
As with Option 2A Stage 2, organisations wishing to provide mitochondrial donation under Option 2B would need to apply for a clinical practice research and training licence and a clinical practice licence.
Given there is only a single licenced clinic in the UK, with a population of over 60 million people, it is possible that only a single clinic will be granted a licence for clinical practice in Australia. However, given the geographical size of Australia, in estimating the potential regulatory costs associated with Option 2B it has been estimated that up to three may apply.
Essentially, Option 2B bypasses Stage 1 of Option 2A and proceeds directly to Stage 2. This approach would result in the immediate implementation of a novel genetic technology into clinical practice, before its safety and effectiveness is fully understood, and the medium and long term effects are not yet known. This would be also be undertaken in circumstances where there are few overseas precedents that could inform the implementation in Australia. This may create risks as to the health of children born of the procedure which could not be mitigated due to the lack of knowledge.
As detailed at Attachment B, the total regulatory costs associated with Option 2B is $141,643 annually or $1,416,426 over ten years. As with Option 2A, this cost will be incurred by businesses.
Overall, given the extremely specialised and technical nature of the procedure and the very small number of couples per annum who would be expected to seek these services, the overarching regulatory impact associated with both Option 2A and 2B is considered minimal compared to the net benefit.
Option 3: Financial assistance for affected parents to seek assistance from UK
Under Option 3, it would be up to prospective parents to determine whether to pursue access to existing services available in the UK, and whether they wished to seek Australian Government assistance to help meet the costs associated with their decision. As such there are regulatory costs for individuals associated with applying for government support and acquitting expenses for any support received.
Option 3, extending the Medical Treatment Overseas Program to people seeking mitochondrial donation would involve direct fiscal costs for the Government. Eligible applicants would be entitled to reimbursement for the costs of overseas hospital accommodation the cost return business class international air fares; other costs including travelling expenses incurred within Australia associated with travel between the applicant's home and the airport, passport fees, travel insurance and departure tax; and non-hospital accommodation expenses.
As detailed at Attachment B, the total regulatory costs associated with Option 3 is $17,920 annually or $179,200 over ten years. This cost will be incurred by individuals.
Assessing the net social benefit
During consultation, some religious groups opposed the introduction of mitochondrial donation on the grounds that it would have a negative impact from a social and ethical perspective. These stakeholders may see a positive net social benefit from Option 1, maintaining the status quo, which could be argued would avoid unintended consequences that might arise from adopting the techniques without further analysis and consideration.
For Option 2, broader impacts to the health sector will be assessed separately prior to mitochondrial donation techniques being approved for clinical practice. Impacts will be assessed using similar criteria to that employed to inform the UK Government's decision. Particularly, a cost benefit analysis was undertaken to estimate the impacts associated with enabling mitochondrial donation treatment to take place, which included financial benefits over time. Specifically, the financial impacts that were quantified were:
- •
- benefits to patients who receive the treatment and their families
- •
- benefits to business providing this service
- •
- reduction of costs to the NHS (the UK Health Service) in provisioning support for the management of mitochondrial disease
- •
- general benefits to the UK economy due to the greater contribution to be expected from healthy people born
- •
- the cost of provisioning mitochondrial donation techniques, and
- •
- the cost of administering regulations supporting mitochondrial donations.
In the Australian context, the impacts that will need to be assessed at the clinical practice phase of this proposal (that is, in approximately 10+ years) will include:
- •
- cost to clinics that will provide the treatment
- •
- costs to individuals seeking approval to have the treatment
- •
- costs to ERLC and other bodies who will regulate clinics/providers of treatment [15]
If clinical application of the research results in fewer children being born with severe mitochondrial disease there will be likely regulatory impact. Reducing the health burden on individuals could also reduce the regulatory burden on medical enterprises as potentially affected children need to interact with the health system less. This impact cannot be measured at this stage, and further analysis will be triggered by a subsequent regulatory decision point post the clinical trial.
Economic and social benefits will also result from people who would otherwise have been suffering illness contributing more actively to the economic and social activity.
As noted above, the economic analysis of the health system and administrative costs of introducing mitochondrial donation vs the predicted health savings which was undertaken in 2014, estimated a net benefit of £32 million (approximately AUD $60 million) per annum if mitochondrial donation enabled the births of just 20 children that year. This estimate did not include savings from reduced social service costs and the possibility for greater workforce participation by parents due to a reduction on caring duties.
The net social benefits associated with Option 3 reflect the same considerations. Supporting affected families to gain access to life-saving technology in the UK will result in financial and infrastructure benefits as fewer people would need to access the Australian health system for treatment of mitochondrial disease.
Potential risks or unintended consequences
Under Option 1 there would be no change to the current system. Prospective parents would have no mechanism for having a biological child with a minimised risk of inheriting mitochondrial disease.
Options 2 and 3 would both include the possibility of unintended consequences, given the newness of the science, and relatively little known including about the long-term effects.
Option 2 would build in follow-up protocols and notification of adverse events to manage risks to trial participants and/or patients and any children born, to identify and further manage the risks of any unintended consequences. Option 2A has more enhanced follow-up than option 2B, as these matters would be considered in depth as part of the Stage 1 clinical trial.
Option 3 would not clearly provide an ongoing mechanism for follow-up, as there is no clinical oversight or follow up expressly included in this option or adverse events notification to Australian regulators or policy makers. This would reduce the ability for Australian regulators and policy makers to have much insight into the safety and effectiveness of the treatments overall, as little detailed information is released about overseas treatment.
In contrast, Option 2 provides the best way of managing unintended consequences, as Australian policy makers and regulators will have insight into the technique and its safety and effectiveness. If unintended consequences were to emerge, it would then be possible to manage these. Option 2A is also better than Option 2B in this regard, as the clinical trial will give Australian policy makers and regulators a good insight into the safety and efficacy of the treatments, before they can be applied in general clinical practice.
Legislative considerations
Under Option 2, amendments would be made to the PHCR Act and the RIHE Act to allow for the following to be undertaken:
- •
- specific research and training using permitted mitochondrial donation techniques
- •
- clinical trials using permitted mitochondrial techniques, and
- •
- eventually clinical practice, once permitted mitochondrial donation techniques have been specified.
The legislation does not however deal with where in Australia the research and training, or the clinical trial, could be carried out. Nor does it deal with the number of persons who can obtain licences for such research and training, or to conduct a clinical trial. However, even once permitted techniques have been prescribed for use in clinical practice, clinical practice will only be possible in a particular state or territory if the state or territory has enacted laws to authorise the practice.
RIS Question 5: Who did you consult and how did you incorporate their feedback?
In 2018, the Senate Community Affairs References Committee undertook an inquiry into the Science of mitochondrial donation and related matters (the Senate Inquiry). [16] The Senate Inquiry looked at the impacts of mitochondrial disease, the science of mitochondrial donation, legal and ethical considerations and regulation. The final report arising from the Senate Inquiry recommended that some further consultation should be undertaken with the community, relevant experts and the states and territories before mitochondrial donation was introduced into Australian clinical practice. [17]
In 2019-20, the NHMRC undertook a series of community and expert consultation activities in response to the Senate Inquiry recommendations [18] . Experts agreed mitochondrial donation may reduce the risk of transmission of mitochondria from the prospective mother and in doing so can prevent future generations from inheriting mitochondria that predispose them to mitochondrial disease. However, these techniques also raise a number of ethical issues that have been identified through public consultation processes undertaken both in Australia and overseas. These include the creation of so-called 'three-parent babies', and concerns regarding heritable genetic modifications, outlined as follows.
Some people are concerned that embryos subject to mitochondrial donation would result in children born through the use of this technology having 'three parents'. However, most experts agree that this is not an accurate description. Children born using this technology still only have two biological parents - a mother and a father and will inherit the majority of their characteristics and personality traits from their biological parents through their nuclear DNA. A female donor involved in a mitochondrial donation process, only provides healthy mitochondria.
This aligns with the findings of the 2018 Senate Inquiry, which concluded that mitochondrial donation techniques do not lead to children having three genetic parents. More importantly, the introduction of this technology will expand the reproductive options for some women and couples affected by severe forms of this disease. It will allow them to have their own biological children, while ensuring that those children will not suffer the devastating consequences of severe mitochondrial disease.
Some people are concerned that mitochondrial donation is a form of genetic engineering, allowing for the creation of 'designer' babies and that, in the future, the technology could be used to modify embryos to produce children with perceived improvements.
Others disagree with the idea that embryos resulting from mitochondrial donation are 'designer' babies, as the mitochondrial DNA donated through this process does not have a meaningful impact on the identity or personal characteristics of the resulting child.
There is also concern that manipulating or altering of genetic material in embryos may result in other unintended (and as yet unknown) consequences. However, legislative amendments will not permit any intentional modification of the mitochondrial DNA or the nuclear DNA.
Mitochondrial donation can involve both the creation and destruction of embryos, depending on the specific technique used. For some people, the creation and destruction of embryos inherent in mitochondrial donation makes the procedure unethical. Concerns centre on denying embryos the chance to live as well as destroying one embryo to allow for the creation of another embryo.
Questions about the moral status of the embryo are not new. Several submitters to the 2018 Senate Inquiry noted that they could see little ethical difference between mitochondrial donation techniques and other procedures that have already been legalised such as pre-implantation genetic diagnosis.
Some experts suggest mitochondrial donation should be limited, at least initially, to the implantation of only male embryos (as the mitochondria are inherited maternally). They argue that this would prevent transmission of the altered genome to future generations, especially in the short term whilst any longer term risks of the procedure are monitored.
This view was not supported in the UK, where sex selection is not considered to be necessary in the context of mitochondrial donation. In Australia, sex selection of embryos is banned unless it is to reduce the risk of transmission of a serious genetic condition. For mitochondrial donation, there may be a case in favour of sex selection. However, there are risks, including that it would reduce the efficiency of the treatment as only half the viable embryos would be usable and would also cross into the existing global controversy about sex selection by couples who prefer male children.
The proposed approach for Australia is to require prospective parents to attend pre-treatment counselling, where the potential risks of the technique would be fully explained. This approach would allow for parents to make their own informed decision, including in relation to sex selection, and provides for reproductive choice.
The wellbeing and rights of the child have also been raised as an area of concern for some members of the community. Mitochondrial donation promotes the health and wellbeing of any resulting children through the prevention of severe mitochondrial disease. However some argue that it will be impossible to protect the interests of children born through this process.
To address this, any legislation will ensure that the privacy of families and children is maintained and that ongoing monitoring will be undertaken through the mainstream health care system with no invasive testing being undertaken for routine monitoring purposes. Mandatory reporting of any adverse events will be required, however individual privacy will be paramount.
Donor rights and responsibilities have also featured in consultation processes undertaken both in Australia and overseas. One issue raised relates to the donor's parental rights to, and responsibilities for, any resulting child and whether the donor may wish to have access to (or make a claim on) any child resulting from her donation. People have also highlighted the need to ensure donors are not exploited nor the provision of eggs incentivised.
To address these issues, it is proposed that donor rights and responsibilities for Australian mitochondrial donation egg donors would be largely aligned to current ART regulations. This would include that:
- •
- Mitochondrial donation egg donors would not be considered legal parents in line with current ART sperm and egg donors under the Family Law Act 1975; and
- •
- Children conceived by mitochondrial donation would have the right to apply for identifying information about their donor when they turn 18 years old.
Submissions to the NHMRC public consultation process found that individuals and organisations representing clinicians and scientists were mostly supportive, although this was generally for a cautious introduction within an appropriate regulatory environment. [19] Patients and advocacy groups were also generally supportive.
Organisations and individuals presenting religious views were generally not supportive. Amongst those opposed, many felt the techniques themselves crossed unacceptable ethical lines. Some felt these techniques created children with three parents or were a form of genetic modification. A number of respondents had concerns regarding the safety of the techniques and the potential for unintended consequences.
For those who supported the introduction of the technology, the benefits of reducing the impacts of mitochondrial disease were seen to outweigh the risks of introducing the technology.
Both the Senate Inquiry and the NHMRC consultation activities identified that that whilst there is support for legalising mitochondrial donation for use in Australia, there is currently not a consensus view. These processes also identified a range of technical scientific, legal and ethical considerations that need to be taken into account in the context of any proposed legislative change.
The range of community and expert views raised during the Senate Inquiry and NHMRC consultations strongly influenced the development of Option 2A, which offers a cautious and staged approach with emphasis on research and a clinical trial, ahead of broader introduction into clinical practice.
In developing Option 2A, the Department of Health consulted within Government, with relevant portfolio agencies (namely, the NHMRC, the Office of the Gene Technology Regulator and the Therapeutic Goods Administration), the Australian Government Solicitor and with the Attorney-General's Department.
The Department also convened an expert panel, with specialist members and consulted with the panel when developing details of the preferred option. The expert panel provided medical, scientific and legal advice on which techniques can and should be approved in Australia, and assisted with developing an implementation pathway and regulatory framework to safely and effectively enact these changes.
Consultation on a staged approach to legalising mitochondrial donation
The views of key stakeholders on whether to legalise mitochondrial donation are well known as a result of previous reviews and consultations. What was less clear was their views in regard to a staged approach to legalising mitochondrial donation, in particular the required amendments to legislation, the potential regulatory framework and the practical aspects associated with implementation.
To help further inform the Government's consideration of the available options, a public consultation paper outlining a staged approach to legalising mitochondrial donation as a pathway to clinical use was released on 5 February 2021 and closed on 15 March 2021.
This consultation provided the opportunity for members of the public, peak bodies, professional stakeholders, and state and territory governments, to consider the merits of a staged approach and inform the development of any legislative amendments and the potential practical implementation of such an approach.
The public consultation process included
- •
- A media release from Minister Hunt on the opening of the consultation period.
- •
- Use of the Department of Health's Consultation Hub for managing the public consultation process.
- •
- A dedicated web page on the Government's proposed approach on the Department of Health website which included: details of the public consultation; a Q & A section and links to the information on the NHMRC website about mitochondrial donation and the outcomes of their public consultation process and reports.
- •
- A dedicated email address for inquiries.
In addition to the release of a discussion paper, the Department of Health also undertook targeted consultations on an exposure draft of the legislation with:
- •
- relevant groups such as the Mito Foundation and religious representatives;
- •
- Commonwealth government agencies;
- •
- Mitochondrial disease experts, researchers and industry groups; and
- •
- state and territory governments, given the PHCR Act and the RIHE Act are part of a uniform legislative scheme, with states and the Australian Capital Territory having passed mirroring legislation.
Those stakeholders contacted directly were provided with the opportunity to meet with senior staff to discuss the proposed approach. Meetings were subsequently held with the Mito Foundation, states and territories and various religious organisations. A panel of scientific and legal experts were extensively consulted in the development of the relevant legislation.
All feedback and submissions from the consultation process were carefully considered in the development of the legislation for allowing mitochondrial donation in Australia. A Consultation Outcomes Report was published on the Department's website shortly after the consultations closed.
A significant proportion of the responses to the Department's online survey were from individuals who had a lived experience or family members with, or who have passed away from, mitochondrial disease. These respondents expressed their full support for the Government in legalising mitochondrial donation. The majority of submissions from respondents who did not have a direct experience of mitochondrial disease still held strong views in support of introducing mitochondrial donation. Some concerns were raised during the consultation process, primarily relating to the destruction of embryos, genetic germline modification, and privacy for patients. Where possible, these issues have been addressed in the legislation.
RIS Question 6: What is the best option from those you have considered?
The overarching net benefit position arising from Options 2 and 3 is that the Government has an obligation to secure and support the health of Australian citizens and ensure, to the extent possible, that babies are not born with severe medical conditions. Therefore, there is an inferred imperative for the Australian Government, if it has access to potentially lifesaving technology, to introduce and develop such clinical practices as a fundamental public good. This would not occur under Option 1, maintaining the status quo.
If Option 3, support to access the UK health system, is pursued it is possible that benefits for the Australian health system and economy could be realised more quickly than under option 2A. That said, the COVID-19 pandemic has disrupted global travel between Australia and the UK significantly, and normal flight services may not be restored for some years. Australian Government support for families to access treatment oversees would be extremely unusual, and is not likely to be supported. The only licensed clinic in the UK that can undertake this technique is also not currently accepting referrals from non-British patients and it is unclear when, if ever, this would become an option for Australian's seeking access to this technology in the UK.
There are also practical advantages for couples in having mitochondrial donation available in Australia, as opposed to having to travel and undergo treatment overseas. These include but are not limited to: financial considerations; the capacity for the parent not undergoing the procedure to continue employment; the possibility that families may have other children or relatives requiring their care in Australia; access to emotional support that would be absent overseas.
Accordingly, Option 2A, a staged approach to legalising mitochondrial donation as a pathway to clinical use, is the preferred option. It is preferred over Option 2B as it responds to community and expert opinion to proceed cautiously, with a focus on further research, safety and efficacy, while still providing for the small number of families seeking to utilise mitochondrial donation now, through the clinical trial. It will also allow for greater knowledge and understanding of the technology, including immediate, medium and longer term risks and how these may be mitigated before this technology is offered more broadly. In addition, given the small number of women expected to seek access to this technology in the first few years, restricting it to an initial clinical trial will not limit access but will assist with building an understanding of the feasibility of introducing this technology more broadly. This approach will also enable eligible women in Australia to access this treatment more quickly.
The establishment of an appropriate regulatory framework will ensure only licenced facilities are able to use these techniques. The creation of separate licences for specific activities will enable close monitoring and ensure that safety and efficacy remain primary considerations as organisations move towards clinical application of approved techniques.
Under Option 2A there is no guarantee that mitochondrial donation will be made available through clinical practice more broadly (Stage 2). Rather this will be a separate decision for Government, based on the outcomes from Stage 1, community and expert views and a detailed consideration of the associated benefits.
Should the Government decide to proceed to clinical application of mitochondrial donation, important knowledge, experience and an evidence base would be lost to Australia if Stage 1 was bypassed. The loss of approximately 10 years' worth of enabling knowledge might manifest as increased risks in the event mitochondrial donation was permitted for clinical use in the short term (that is, without a clinical trial).
Medical tourism is another associated advantage with Option 2 (whether 2A or 2B), however this is not the reason for pursuing introduction of mitochondrial donation in Australia. Investing in a period of research and training may see Australia become an expert hub in the region and a centre for medical tourism if neighbouring countries don't adopt similar techniques.
Based on available data, the medical tourism market in Australia is small and scattered. A 2011 study by Deloitte Access Economics found that in 2010, visitors for medical reasons (around 12,800 people) comprised only 0.23 per cent of total visitors in Australia - around 5.5 million people. [20] At this stage it is impossible to predict how these numbers would increase should Australia become a hub of expertise in mitochondrial donation techniques, but it would be reasonable to expect these figures to increase, particularly if Australia becomes a desired medical destination for people from the United States and New Zealand, two potential source markets identified in the Deloitte study.
Benefits arising from medical tourism with regard to expenditure and enhanced practical knowledge and training would clearly not be realised under Options 1 and 3. Whereas there would be benefits under Option 2B, the more cautious monitoring process and skills development under Option 2A creates safeguards that would likely enhance Australia's attraction as a medical destination for overseas patients.
RIS Question 7: How will you implement and evaluate your chosen option?
The preferred option would be implemented in the following staged manner:
1. Implementation - Stage 1 (10+ years)
Under Stage 1 the Australian Government will amend the PHCR Act and the RIHE Act to legalise mitochondrial donation for certain research and training purposes, and to support selection and licensing of the first clinic for delivery of mitochondrial donation to impacted families.
The use of specified mitochondrial donation techniques under Stage 1 will be subject to strict licensing conditions, which would be overseen by the existing ERLC of the NHMRC.
Initially Stage 1 will include implementing any administrative changes to expand the current role and remit of the ERLC in line with the amendments to the RIHE Act, under which this committee is established. This will include establishing required administrative arrangements for licensing and oversight of mitochondrial donation licences, including for research and training licences, and clinical trial licences required to create human embryos using the mitochondrial donation techniques permitted for each licence as well as approval processes for individuals seeking treatment. The Commonwealth Department of Health portfolio will also undertake a competitive grants process to identify a suitable organisation to undertake the closely monitored clinical trial.
It is expected that Stage 1 will take around 10 years, based on the potential for participants to require multiple IVF procedures before a successful pregnancy is achieved.
Throughout Stage 1, ongoing monitoring and evaluation will occur.
2. Implementation - Stage 2
Clinical practice using approved mitochondrial donation techniques under Stage 2 would commence only after the RIHE Regulations have been amended to specify mitochondrial donation techniques that are suited to be used in clinical practice. This decision will be based on the clinical trial progress and outcomes and other expert advice.
State and territory governments will be able to opt-in to the national regulatory framework if they wish. Clinics within those jurisdictions will then be able to apply for a licence via the ERLC to offer mitochondrial donation as part of clinical practice.
The amendments to the PHCR Act and the RIHE Act to implement Option 2 in this manner would be reviewed periodically, every 7 years.
Models of care
There are several models of care that could be used by the organisation selected for the clinical trial to deliver mitochondrial donation to families. Equity of access, and the impact on families as they participate in the clinical trial have been considered, as has the requirements of each party (mother, father and donor) from a medical perspective.
Practically, the trial is likely to require a partnership approach which would be led by a research organisation with significant mitochondrial disease and research expertise that would be responsible for:
- •
- administering the trial, supporting an appropriate governance structure, data collection and management, undertaking research and regular progress reporting, including reporting of adverse events;
- •
- licence applications and ongoing compliance with licensing requirements;
- •
- trial participant application processes including providing he clinical and research expertise and support to assist the ERLC to assess individual participant approvals (through the establishment of an independent Clinical Advisory Committee);
- •
- managing and coordinating with the relevant partner organisations to manage ethics approvals and ensuring all research is conducted in accordance with all Australian regulatory, legal and ethical frameworks; and
- •
- developing overarching processes and protocols for working with families, including consent protocols and pre-treatment counselling, and ensuring a consistent approach and experience for families across the clinical trial partner organisations.
Access to a specialist diagnostic lab for genetic and pathology diagnosis would also be required to support application processes for participation in the trial as well as specialist mitochondrial disease clinics responsible for referral, pre-treatment counselling, and ongoing clinical support of the families participating in the trial.
Service delivery for the IVF and mitochondrial donation techniques would need be provided through a specialist IVF provider. Ideally families will be able to access some or all of the required services in the capital city closest to them.
The Department of Health will be responsible for ongoing project management and regular review of the legislation.
Mitochondrial Donation Donor Register
The Department of Health would also establish and manage a confidential Mitochondrial Donation Donor Register (the Register). The purpose of this register is to provide children born as a result of mitochondrial donation the option to apply for identifying information about their mitochondrial donor, after they reach the age of 18 years. Mitochondrial donors would also be able to request to review their own information on the Register for the purposes of updating that information should they wish to do so. Donors would not however be able to access information about any children born or any other details on the Register.
It will be an offence to disclose information on this Register, unless it is for the purpose of disclosing to the child born as a result of a mitochondrial donation technique after they turn 18, a mitochondrial donor for the purposes of updating their own information, or by order of a court.
ATTACHMENT A
Proposed regulatory framework to support legalising mitochondrial donation as a pathway to clinical use
In the case of Option 2A, in Stage 1, organisations wishing to undertake a clinical trial using mitochondrial donation techniques for human reproductive purposes, will be required to apply to the ERLC for two separate, consecutive licences including an initial clinical trial research and training licence followed by a clinical trial licence. To receive Government funding for this trial, the organisation will also need to apply for and be successfully awarded a Commonwealth clinical trial grant.
Note the pre-clinical research and training licence is separate, and is for a limited range of lab-based research only which is not conducted for the purposes of a particular clinical trial and which can only be undertaken using mitochondrial donation techniques permitted for this purpose under the regulations.
The clinical trial research and training licence will permit research and training to be undertaken using techniques that have been approved for use in a clinical trial, currently MST and PNT, with a view to these techniques being applied in a clinical trial setting.
It will not be possible to obtain this licence for the use of other techniques that have not been specified under the regulations as approved for use in a clinical trial.
The licence will be for research and training (as specified in the legislation) with a view to:
- •
- determining the safety and efficacy of using the licenced clinical mitochondrial donation technique in preventing the transmission of serious mitochondrial disease
- •
- developing and refining protocols for the safe and effective use of mitochondrial donation techniques in a clinical trial setting
- •
- training staff to perform the technique in a clinical setting, and
- •
- establishing suitable facilities within which the technique can be safely and feasibly performed.
It is expected that only a small number of organisations (1 - 3) will be interested in undertaking the clinical trial. It is also intended that Commonwealth funding will be approved for one clinical trial under Stage 1. Therefore, only one organisation will be likely to apply for a clinical trial research and training licence.
The approved clinical trial organisation will then need to apply for a clinical trial licence before proceeding with the Commonwealth funded clinical trial. This licence will permit the conduct of a clinical trial (for human reproductive purposes) using MST and/or PNT. A clinical trial licence can only be obtained if the licence applicant has met all the conditions required as specified in the legislation.
In addition, each individual woman seeking treatment under the clinical trial, will be required to seek approval to participate, through the ERLC. The organisation undertaking the clinical trial will be responsible for obtaining the information required by the ERLC to be able to assess the eligibility of each individual to participate in the trial. This will include, but will not be limited to, a range of clinical advice, including the particular risk of the women's offspring inheriting mitochondrial disease, the severity of the disease or illness that would be inherited, whether other available techniques could be used to reduce the risk and whether the woman and her spouse (if any) had attended pre-treatment counselling.
A third category of licence will also be available under Stage 1, a pre-clinical research and training licence. This licence will permit research and training to be undertaken for the purposes of building evidence and expertise in recognised mitochondrial donation techniques to ensure the most effective technique is able to be used in the future, using permitted techniques specified under regulations. This licence will permit research and training to be undertaken for non-reproductive purposes. It is expected that only a very small number of organisations (up to 5) may wish to apply for this type of licence. Organisations wishing to apply for a 'pre-clinical research and training' licence will be required to apply to the ERLC for a licence.
Under Stage 2, any clinics in states and territories that opt-in to the national regulatory framework, who wish to offer mitochondrial donation will be required to apply to the ERLC for a 'clinical practice research and training' licence and 'clinical practice' licence. In addition, the individuals wanting to access the procedure, will need to seek ERLC approval.
ATTACHMENT B
Calculation of the costs associated with each option
Option 1: Maintain the status quo
There are no regulatory costs associated with this option.
Table 1: Regulatory Burden Estimate for Option 1
| Average Annual Regulatory Costs (from business as usual) | ||||
| Change in Costs ($ million) | Business | Community Organisations | Individuals | Total change in cost |
| Total by sector | $0 | $0 | $0 | $0 |
Option 2A : Research and clinical trial
Under Stage 1, the primary regulatory costs for entities wanting to build expertise in mitochondrial donation will be associated with the need to obtain (and maintain, where relevant) the required licences.
While some of the details and principles of the Australian regulatory framework and licensing requirements for mitochondrial donation will be provided for under the legislation, further detailed licensing requirements will be developed by the ERLC as their role is expanded following enactment of the proposed legislation. However, it is anticipated that it will closely follow the existing ERLC licensing requirements for the use of excess ART embryos and the UK HFEA licensing requirements. Based on the advice of the NHMRC this is estimated to take an organisation around 40 hours to complete.
Given the complexity of the application and compliance processes, it is assumed that both administrative personnel and clinical experts will need to be involved. Following advice from the Office of Best Practice Regulation (OBPR) the default labour cost of $73.05 per hour (including on-costs) has been used to calculate the 'administrative officer' component. [21] The equivalent cost for a clinical expert, based on recent Department of Health experience, is $1000 per hour (including on-costs). The following costings have been prepared on the assumption that they will both complete 50 per cent of each licence application process.
It is assumed that entities applying for any of the licences will have suitable facilities and expertise, and that they will not need to incur additional capital or staffing costs as a direct consequence of the regulatory framework. Organisations holding any of the licence types will also incur some regulatory costs associated with maintaining the conditions of the licence which have been estimated to be equivalent to that of the initial application.
Licences will be required for any type of mitochondrial donation research and training, clinical trial or clinical practice, as detailed above. Under Stage 1 these licences would include, a:
- •
- pre-clinical research and training licence;
- •
- clinical trial research and training licence; and
- •
- clinical trial licence.
Under Stage 2 (clinical practice) these licences would include, a
- •
- clinical practice research and training licence; and
- •
- clinical practice licence.
Regulatory costs for applying for a pre-clinical research and training licence would involve no additional regulatory cost as requirements associated with applying for this licence would be the same as for any current application to the ERLC for other embryonic research.
The regulatory cost of applying for the clinical trial research and training licence
Initially, an organisation proposing to undertake a specific clinical trial would need to apply for a clinical research and training licence to enable that organisation to develop clinical and patient protocols and to build technical expertise and proficiency in using the techniques to create viable human embryos. On the assumptions outlined above, an individual facility is likely to expend around $21,460 applying for such this licence. The expectation is that only one organisation will apply for this licence.
While the organisation would be required to expend this cost upfront in completing the licence application, it is anticipated that this expense would be reimbursed by the Commonwealth through the provision of funding under a clinical trial grant.
The organisation would also incur annual compliance costs associated with maintaining the conditions of the licence, for instance meeting the reporting requirements to ERLC. The yearly compliance cost is estimated to be equivalent to the initial licence application cost.
Annualised over ten years the total cost of the licence would be $23,607, as calculated in Table 2 below.
Table 2: Calculating the regulatory cost of the clinical trial research and training licence
| Assumption | Calculation | Cost ($) |
| Application | ||
| 20 hours of administrative officer | 20 x $73.05 | 1,461 |
| 20 hours of clinical expert | 20 x $1000 | 20,000 |
| Labour cost per licence application | 21,461 | |
| Compliance | ||
| 20 hours of administrative officer | 20 x $73.05 | 1,461 |
| 20 hours of clinical expert | 20 x $1000 | 20,000 |
| Yearly cost to comply with licence | 21,461 | |
| 10 Year cost | ||
| Application | 1 x 21,461 | 21,461 |
| Compliance | 10 x 21,461 | 214,610 |
| 236,071 | ||
| Yearly cost over 10 years | 236,071 / 10 | 23,607 |
The regulatory cost of applying for the clinical trial licence
A clinical trial licence would be needed by the facility approved to undertake the clinical trial. As with the clinical trial research and training licence, the expectation is that a clinical trial licence application will take around 40 hours to complete, with input shared between administrative and clinical experts.
It is expected that only one facility will receive Commonwealth funding to undertake the clinical trial and therefore only one organisation will be eligible to apply for a clinical trial licence, making the annualised regulatory cost of this licence over ten years $23,607, as calculated in Table 3 below.
Table 3: Cost of the clinical trial licence
| Assumption | Calculation | Cost ($) |
| Application | ||
| 20 hours of administrative officer | 20 x $73.05 | 1,461 |
| 20 hours of clinical expert | 20 x $1000 | 20,000 |
| Labour cost per licence application | 21,461 | |
| Compliance | ||
| 20 hours of administrative officer | 20 x $73.05 | 1,461 |
| 20 hours of clinical expert | 20 x $1000 | 20,000 |
| Yearly cost to comply with licence | 21,461 | |
| 10 Year cost | ||
| Application | 1 x 21,461 | 21,461 |
| Compliance | 10 x 21,461 | 214,610 |
| 236,071 | ||
| Yearly cost over 10 years | 236,071 / 10 | 23,607 |
The estimated total regulatory cost associated with Stage 1, which is the summation of the regulatory costs for applying for and complying with the two clinical trial related Stage 1 licences, is $472,142 over 10 years, or $47,214 annually.
With Stage 1 likely to last around 10-12 years, and the decision on whether to proceed to Stage 2 dependent on the outcomes of Stage 1 the estimated regulatory impacts for Stage 2 provided below will need to be reviewed prior to transition to this Stage.
The regulatory cost of applying for the clinical practice research and training licence
A clinical practice research and training licence is the initial licence required by organisations wishing to provide mitochondrial donation in a clinical setting. This licence will allow organisations to develop clinical and patient protocols and to build technical expertise and proficiency in using the techniques to create viable human embryos. It is assumed that up to three organisations may wish to apply for this licence. The application process is expected to have the same variables as above, ie take around 40 hours to complete, by administrative and clinical personnel.
The estimated regulatory cost of the clinical practice research and training licence is $70,821 annually or $708,210 over 10 years. Table 4 outlines how this cost has been calculated.
Table 4: Calculating the regulatory cost of the clinical practice research and training licence
| Assumption | Calculation | Cost ($) |
| Application | ||
| 20 hours of administrative officer | 20 x $73.05 | 1,461 |
| 20 hours of clinical expert | 20 x $1000 | 20,000 |
| Labour cost per licence application | 21,461 | |
| 3 facilities apply | 3 x $21,461 | 64,383 |
| Compliance | ||
| 20 hours of administrative officer | 20 x $73.05 | 1,461 |
| 20 hours of clinical expert | 20 x $1000 | 20,000 |
| Yearly compliance cost per licence | 21,461 | |
| 3 facilities licensed | 3 x $21,461 | 64,383 |
| 10 Year cost | ||
| Application | 3 x 21,461 | 64,383 |
| Compliance | 10 x 64,383 | 643,830 |
| 708,213 | ||
| Yearly cost over 10 years | 708,213 / 10 | 70,821 |
Once proficiency in using the technology has been established and appropriate clinical and patient protocols for providing mitochondrial donation in a clinical practice setting have been developed, each organisation would then need to apply for a clinical practice licence before the technique could be offered to prospective mothers.
The regulatory cost of applying for the clinical practice licence
A clinical practice licence will be required by any organisation wishing to provide mitochondrial donation in a clinical setting. It is assumed that all three organisations holding a clinical practice research and training licence would subsequently apply for a clinical practice licence. The application process is expected to have the same variables as above, ie take around 40 hours to complete, by administrative and clinical personnel.
The estimated regulatory cost of the clinical practice licence is $70,821 annually or $708,213 over 10 years. Table 5 outlines how this cost has been calculated.
Table 5: Calculating the regulatory cost of the clinical practice licence
| Assumption | Calculation | Cost ($) |
| Application | ||
| 20 hours of administrative officer | 20 x $73.05 | 1,461 |
| 20 hours of clinical expert | 20 x $1000 | 20,000 |
| Labour cost per licence application | 21,461 | |
| 3 facilities apply | 3 x $21,461 | 64,383 |
| Compliance | ||
| 20 hours of administrative officer | 20 x $73.05 | 1,461 |
| 20 hours of clinical expert | 20 x $1000 | 20,000 |
| Yearly compliance cost per licence | 21,461 | |
| 3 facilities licensed | 3 x $21,461 | 64,383 |
| 10 Year cost | ||
| Application | 3 x 21,461 | 64,383 |
| Compliance | 10 x 64,383 | 643,830 |
| 708,213 | ||
| Yearly cost over 10 years | 708,213 / 10 | 70,821 |
The estimated total regulatory cost associated with Stage 2, which is the summation of the regulatory costs for applying for and complying with the two Stage 2 licences is $1,416,426 over 10 years, or $141,643 annually.
The estimated total regulatory cost associated with Option 2A (Stage 1 + Stage 2) is $1,888,568 over ten years, or $188,857 annually.
As identified in Table 6 below it will be businesses who incur the regulatory costs associated with Option 2A.
Table 6: Regulatory Burden Estimate Table for Option 2A
| Average Annual Regulatory Costs (from business as usual) | ||||
| Change in Costs ($ million) | Business | Community Organisations | Individuals | Total change in cost |
| Total by sector | $0.19 | $0 | $0 | $0.19 |
Option 2B : Straight to clinical practice
In the UK, with a population of over 60 million people, there is only a single clinic licenced to undertake mitochondrial donation. It is possible that only a single clinic will be granted a licence in provide the service in Australia, however it is estimated that up to three may apply.
As outlined above for Stage 2, each organisation would need to apply for an initial clinical practice research and training licence, followed by a clinical practice licence. Both application processes are expected to have the same variables as above, ie take around 40 hours to complete, by administrative and clinical personnel.
The regulatory costs of these two licences are the same as presented in Stage 2 under Option 2A. For transparency, the calculations are presented again below in Tables 7 and 8.
Table 7: Calculating the regulatory cost of the clinical practice research and training licence
| Assumption | Calculation | Cost ($) |
| Application | ||
| 20 hours of administrative officer | 20 x $73.05 | 1,461 |
| 20 hours of clinical expert | 20 x $1000 | 20,000 |
| Labour cost per licence application | 21,461 | |
| 3 facilities apply | 3 x $21,461 | 64,383 |
| Compliance | ||
| 20 hours of administrative officer | 20 x $73.05 | 1,461 |
| 20 hours of clinical expert | 20 x $1000 | 20,000 |
| Yearly compliance cost per licence | 21,461 | |
| 3 facilities licensed | 3 x $21,461 | 64,383 |
| 10 Year cost | ||
| Application | 3 x 21,461 | 64,383 |
| Compliance | 10 x 64,383 | 643,830 |
| 708,213 | ||
| Yearly cost over 10 years | 708,213 / 10 | 70,821 |
Table 8: Calculating the regulatory cost of the clinical practice licence
| Assumption | Calculation | Cost ($) |
| Application | ||
| 20 hours of administrative officer | 20 x $73.05 | 1,461 |
| 20 hours of clinical expert | 20 x $1000 | 20,000 |
| Labour cost per licence application | 21,461 | |
| 3 facilities apply | 3 x $21,461 | 64,383 |
| Compliance | ||
| 20 hours of administrative officer | 20 x $73.05 | 1,461 |
| 20 hours of clinical expert | 20 x $1000 | 20,000 |
| Yearly compliance cost per licence | 21,461 | |
| 3 facilities licensed | 3 x $21,461 | 64,383 |
| 10 Year cost | ||
| Application | 3 x 21,461 | 64,383 |
| Compliance | 10 x 64,383 | 643,830 |
| 708,213 | ||
| Yearly cost over 10 years | 708,213 / 10 | 70,821 |
The estimated total regulatory cost associated with Option 2B is equivalent to Stage 2 under option 2A (that is the cost associated with the two clinical practice licences). That is $1,416,426 over ten years, or $141,643 annually.
As identified in Table 9 below it will be businesses who incur the regulatory costs associated with Option 2B.
Table 9: Regulatory Burden Estimate Table for Option 2B
| Average Annual Regulatory Costs (from business as usual) | ||||
| Change in Costs ($ million) | Business | Community Organisations | Individuals | Total change in cost |
| Total by sector | $0.14 | $0 | $0 | $0.14 |
Option 3 : Financial assistance for affected parents to seek assistance from UK
It would be up to prospective parents to determine whether they would want to pursue access to existing services available in the UK, and whether they wished to seek Australian Government assistance to help meet the costs associated with their decision. As such there are regulatory costs for individuals associated applying for government support.
A maximum of 56 families (the number of Australian children born annually with severe mitochondrial disease) could apply for support each per year. The actual number of applicants would almost certainly be fewer, as the technology available would only assist families where the disease is caused by the inherited mitochondria themselves. For many children born with mitochondrial disease, this stems from the nuclear DNA and mitochondrial donation techniques would not have prevented the disease.
The application and acquittal process is expected to take around 10 hours to complete, by each family.
Each application would require a specialist referral. But as each applicant family would almost certainly be receiving specialist advice already, this cost should be regarded as 'business as usual' and not considered additional to accessing the support scheme.
Figure 10: Regulatory cost of accessing Australian Government assistance for access to existing UK services
| Assumption | Calculation | Cost ($) |
| Application | ||
| 5 hours to complete each application | 5 x $32 | 160 |
| Acquittal | ||
| 5 hours to acquit each grant | 5 x $32 | 160 |
| Total labour costs per application | 10 x $32 | 320 |
| 56 families apply | 56 x $320 | 17,920 |
| Cost over 10 years | $17,920 x 10 | 179,200 |
The estimated total regulatory costs associated with Option 3 is $179,200 over ten years, or $17,920 annually.
While not visible in Table 11 below due to the low level of the cost incurred under this option, it will be families that meet the associated costs of Option 3.
Table 11: Regulatory Burden Estimate Table for Option 3
| Average Annual Regulatory Costs (from business as usual) | ||||
| Change in Costs ($ million) | Business | Community Organisations | Individuals | Total change in cost |
| Total by sector | $0 | $0 | $0.0 | $0.0 |
